A Research Informed Consent Form is essential for ethically conducting studies, ensuring participants fully understand the purpose, risks, and benefits of their involvement. This guide explores how to create a Consent Form that complies with ethical standards and legal requirements. You’ll learn how to tailor a Research Consent Form to meet study-specific needs, covering key sections like participant details, data usage policies, and withdrawal rights. Whether you’re conducting academic research or clinical trials, this comprehensive guide provides examples and tips for creating clear, concise, and effective forms. Empower your research with informed participant consent today.
Download Research Informed Consent Form Bundle
What is Research Informed Consent Form?
A Research Informed Consent Form is a document that educates participants about a study’s purpose, risks, benefits, and procedures. It ensures participants voluntarily agree to join a study, understanding all aspects involved. This is a critical ethical and legal requirement in research, protecting participants’ rights while enhancing transparency. It often includes details about confidentiality, data usage, and the right to withdraw. These forms promote trust between researchers and participants, ensuring mutual understanding and respect throughout the study.
Research Informed Consent Format
Title of Study:
- [Research Project Title]
Principal Investigator:
- Name: [Investigator’s Full Name]
- Contact Information: [Email and Phone Number]
Purpose of the Research:
- [Detailed description of the research objective and significance.]
Procedures:
- [Step-by-step explanation of what participants will undergo during the study.]
Duration of Participation:
- [Expected time commitment for participants.]
Risks and Benefits:
- [List potential risks and the benefits of participation.]
Confidentiality:
- [Explain how participant data will be stored, used, and protected.]
Voluntary Participation:
- [State that participation is voluntary and can be withdrawn anytime.]
Contact Information for Questions:
- [Provide details of whom to contact for further inquiries or concerns.]
Consent Statement:
- “I have read the above information, and I consent to participate in the research.”
Participant Signature:
- [Signature, Full Name, and Date]
Informed Consent Form for Research Study

An Informed Consent Form ensures participants understand research objectives, risks, and rights. It promotes ethical practices, safeguarding their autonomy and ensuring voluntary participation for transparency and legal compliance.
Clinical Research Informed Consent Form

A clinical research consent form outlines study details, ensuring participants understand potential risks and benefits. Like a Field Trip Consent Form, it formalizes agreement while prioritizing safety and ethical standards.
Psychology Research Informed Consent Form

Psychology research consent forms ensure participants comprehend study goals and confidentiality. Similar to a Photo Consent Form, it seeks explicit permission for ethical engagement and data use.
Research Participant Informed Consent Form

A research participant consent form ensures clear understanding of procedures and rights. It’s essential, like a Minor Travel Consent Form, for protecting participants and establishing transparency between parties involved.
Browse More Research Informed Forms
Research Informed Consent Form Sample
Research Subject Informed Consent Form
Clinical Research Participant Consent Form
College Research Informed Consent Form
Human Subjects Research Informed Consent Form
Observational Research Informed Consent Form
Research Informed Consent Form in DOC
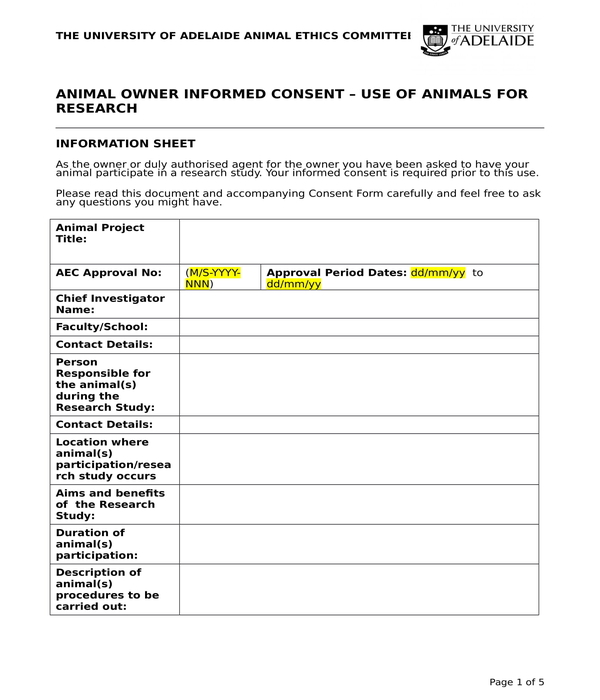
Research Animal Owner Informed Consent Form
Research Participation Informed Consent Form
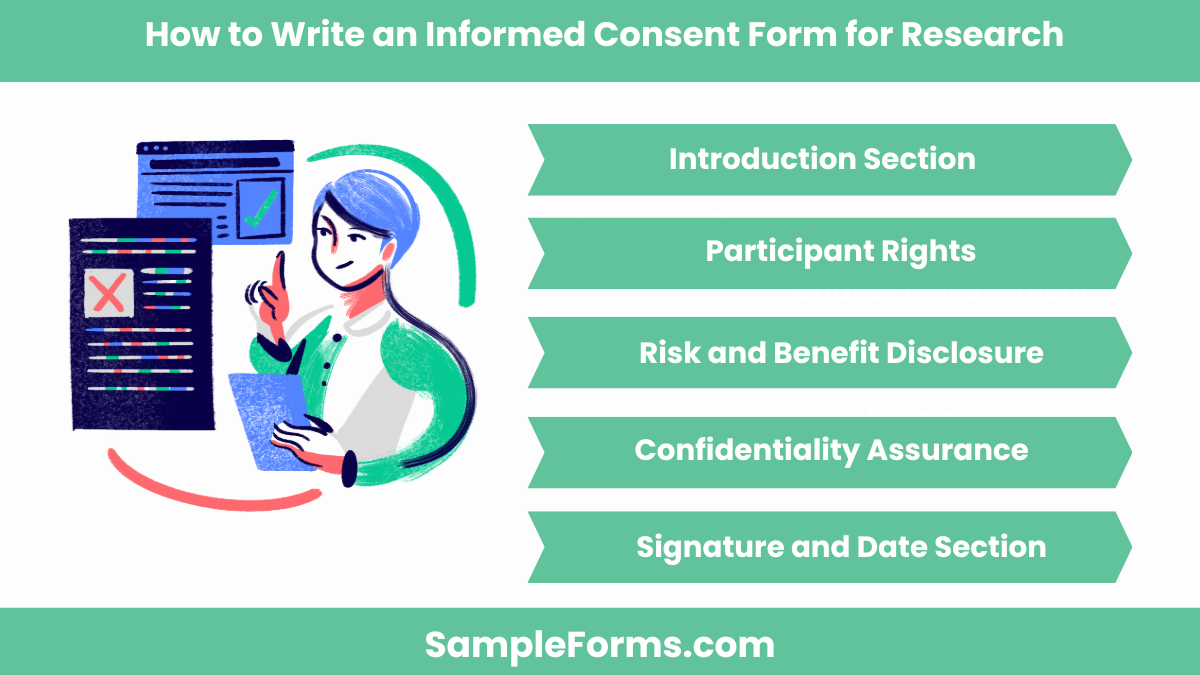
How do you write an informed consent form for research?
Creating an informed consent form ensures participants understand research goals, risks, and rights. Steps for a comprehensive form include:
- Introduction Section: Explain the study’s purpose and objectives in clear, simple language for participants to understand.
- Participant Rights: Outline rights, such as voluntary participation and the ability to withdraw without penalties.
- Risk and Benefit Disclosure: Detail potential risks and benefits to inform participants adequately.
- Confidentiality Assurance: Specify how data will be stored, shared, and protected to maintain privacy, akin to a Medical Consent Form.
- Signature and Date Section: Include fields for participant signatures, affirming their informed and voluntary participation.
What happens if you don’t get informed consent in research?
Failure to obtain informed consent violates ethical and legal standards. This may lead to disputes, penalties, or invalid study results. Key implications include:
- Ethical Violations: Research may be considered unethical, damaging reputations and professional credibility.
- Legal Consequences: Breaches can result in lawsuits or fines, similar to Passport Consent Form misuse.
- Participant Harm: Participants may experience unanticipated risks without informed agreement.
- Data Invalidity: Research findings may be deemed unreliable or unusable without consent documentation.
- Loss of Trust: Lack of transparency erodes trust between researchers and participants.
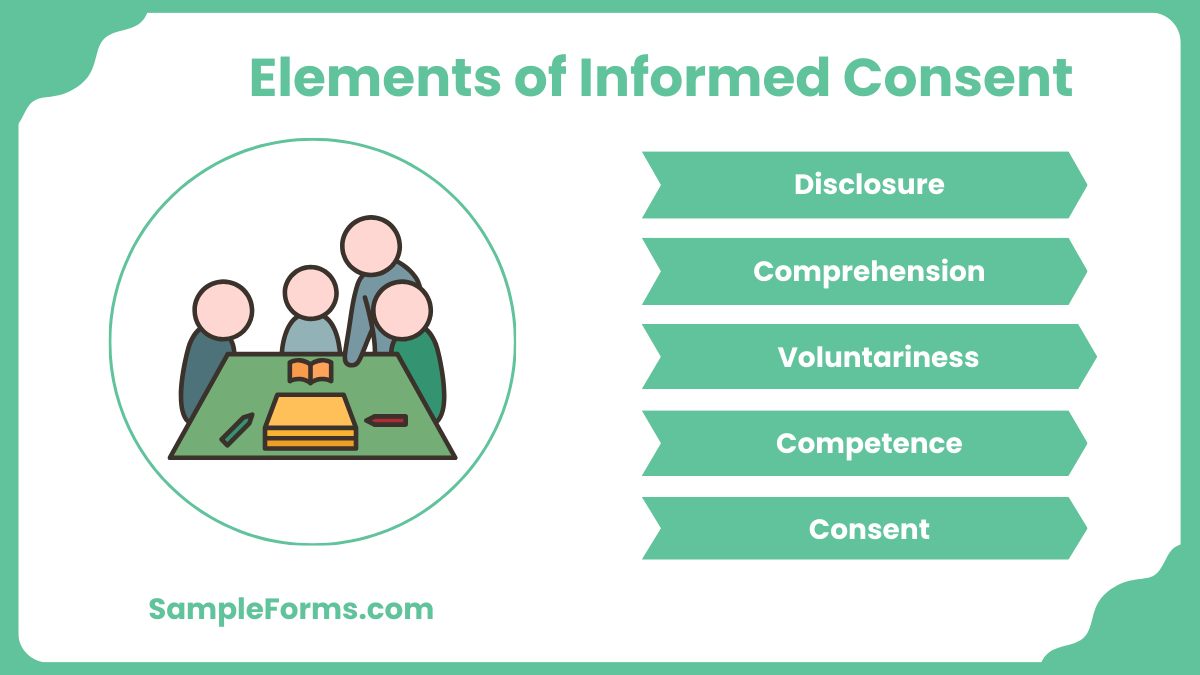
What are the 5 elements of informed consent?
The essential elements ensure participants make informed decisions about research participation. These elements are vital for ethical compliance:
- Disclosure: Provide complete information about the study, including purpose, procedures, risks, and benefits.
- Comprehension: Ensure participants fully understand the information provided, adjusting for language or literacy barriers.
- Voluntariness: Participation must be entirely voluntary without coercion, like in a Child Travel Consent Form.
- Competence: Verify that participants are legally and mentally capable of making informed decisions.
- Consent: Obtain explicit consent, documented through signatures or other formal acknowledgment.
What are the criteria legally necessary in informed consent?
Informed consent requires adherence to legal standards ensuring validity and participant protection. The three criteria include:
- Capacity to Consent: Participants must be mentally and legally capable of providing informed agreement.
- Information Disclosure: Researchers must disclose all pertinent details, ensuring clarity and understanding.
- Voluntary Agreement: Consent must be given freely, without pressure, akin to a Parent Consent Form process.
What are the pillars of informed consent?
The four pillars form the foundation of ethical and legal informed consent processes, promoting participant safety and study integrity:
- Autonomy: Respect participants’ rights to make independent, informed decisions about their involvement.
- Beneficence: Ensure the research provides benefits while minimizing harm, as emphasized in a Business Consent Form.
- Non-Maleficence: Avoid causing harm to participants through research actions or omissions.
- Justice: Treat all participants fairly, ensuring equal access to research opportunities and protections.
Can you conduct research without informed consent?
Research without informed consent is unethical unless legally exempt. For example, emergency studies may waive a Therapy Consent Form requirement under specific conditions.
What is an example of a lack of informed consent in research?
An example includes participants being unaware of study risks or goals, as in cases where a Student Consent Form wasn’t provided for informed agreement.
How to prove lack of informed consent?
Lack of informed consent can be proven by showing incomplete or misleading information, such as omitting risks in a Research Proposal or failing to provide participant options.
Does informed consent have to be written?
Not always. While written consent is preferred, verbal consent may be valid in certain cases, such as filling a Market Research Form under informal agreements.
Which states have implied consent laws?
Many states enforce implied consent laws, especially for procedures like DUI testing. However, formal agreements like a Research Proposal Form often require explicit consent.
What should a consent form include?
A consent form must include study objectives, risks, benefits, confidentiality, and withdrawal rights, similar to an Interview Consent Form structure.
What is a short form consent document?
A short form consent document briefly summarizes participant rights and study details, such as a Surgical Consent Form, ensuring comprehension without extensive details.
What is required for research informed consent?
Research informed consent requires clarity on goals, risks, benefits, and rights, much like what’s essential in a Counseling Consent Form for ethical compliance.
Is informed consent written or verbal?
It can be either, depending on the situation. However, written formats, such as a Child Medical Consent Form, provide better legal protection.
Is there a time limit on informed consent?
Informed consent often requires renewal if circumstances change, as in legal agreements like a Landlord Consent Form, ensuring up-to-date understanding.
A Research Informed Consent Form ensures ethical participant engagement in studies, outlining risks, benefits, and withdrawal rights. By following clear guidelines, researchers create trust and transparency. Examples like the Emergency Consent Form highlight the importance of tailored approaches for specific situations. These forms are pivotal in protecting participant rights while upholding legal and ethical standards. Whether drafting for clinical research or academic purposes, informed consent forms remain a cornerstone of responsible research practices, fostering mutual respect and clear communication between researchers and participants.
Related Posts
-
Minor Travel Consent Form
-
FREE 7+ Drug-Alcohol Testing Consent Forms in PDF | MS Word
-
FREE 8+ Botox Consent Forms in PDF | MS Word
-
FREE 6+ Waxing Consent Forms in PDF | MS Word
-
Surgical Consent Form
-
Field Trip Consent Form
-
FREE 6+ Caregiver Consent Forms in PDF | MS Word
-
Tattoo Consent Form
-
FREE 6+Sample Survey Consent Forms in MS Word | PDF
-
FREE 10+ Check Consent Forms in PDF | Ms Word
-
Parental Consent Form
-
FREE 6+ Model Consent Forms in MS Word | PDF
-
FREE 7+ Participant Consent Forms in MS Word | PDF
-
FREE 7+ Therapy Consent Forms in MS Word | PDF
-
12+ Photo Consent Form